ART: Step-by-Step Guide
Every cycle of ART involves multiple steps, and each occurs at a specific time during a two to six-week period. The following is an overview of the IVF procedure. The procedure begins in the month preceding the actual ART cycle. An IVF cycle typically includes the following steps or procedures:
- Medications to grow multiple eggs
- Retrieval of eggs from the ovary or ovaries
- Insemination of eggs with sperm
- Culture of any resulting fertilized eggs (embryos)
- Placement ("transfer") of one or more embryo(s) into the uterus either during the same cycle as the ovarian stimulation cycle or during another cycle (frozen embryo transfer cycle)
- Support of the uterine lining with hormones to permit and sustain pregnancy
In certain cases, these additional procedures can be employed:
- Intracytoplasmic sperm injection (ICSI) to increase the chance for fertilization
- Assisted hatching of embryos to potentially increase the chance of embryo attachment ("implantation")
- Preimplantation Genetic Testing (PGT)
- Embryo cryopreservation (freezing)
The success of IVF largely depends on growing multiple eggs at once
- Injections of the natural hormones FSH and/or LH (gonadotropins) are used for this purpose
- Additional medications are used to prevent premature ovulation
- An overly vigorous ovarian response can occur, or conversely an inadequate response.
Cycle Preceding ART Cycle
- Sometimes oral contraceptives begin.
- Sometimes a GnRH agonist (e.g. Lupron®) is initiated.
- Sometimes a mock transfer is performed to identify potential problems in embryo transfer.
- An IVF cycle starts with a cycle day of the menstrual period without other preceding medications.
ART Cycle
- Ovarian stimulation with gonadotropins (e.g. Menopur®, Follistim®, Gonal-F®, Follistim AQ pen, and/or Gonal-F RFF Pen)
- Monitoring follicle development with ultrasound and serum estradiol levels
- Final oocytes maturation and induction of the artificial ovulatory surge with either Lupron or hCG administration ( Pregnyl®, Novarel® or Ovidrel®)
- Transvaginal ultrasound guided oocyte retrieval
- Fertilization of eggs either via standard fertilization or ICSI
- Embryo transfer -if planning fresh embryo transfer, accompanied by progesterone supplementation and pregnancy test within 2 weeks of the embryo transfer
- Cryopreserve embryos
Step 1 – Prestimulation Treatment
Initiation of Oral Contraceptives
Some patients will receive oral contraceptives in the cycle prior to the ART cycle. This helps with cycle timing. There is also evidence that oral contraceptives can help prevent ovarian cysts.
Suppression of Ovulation
There are two principle ways that physicians ensure ovulation does not occur before egg retrieval. One involves pre-treatment of a patient with a GnRH agonist. The other involves treatment after six or so days of stimulation with a GnRH antagonist.
GnRH Agonist Administration
GnRH agonist (Lupron®): This medication is taken by injection. There are two forms of the medication: A short acting medication requiring daily injections and a long-acting preparation lasting for 1-3 months. The primary role of this medication is to prevent a premature LH surge, which could result in the release of eggs before they are ready to be retrieved. Since GnRH-agonists initially cause a release of FSH and LH from the pituitary, they can also be used to start the growth of the follicles or initiate the final stages of egg maturation.
A GnRH agonist might be prescribed sometime after taking oral contraceptive pills. This dose may be reduced when ovarian stimulation is begun. Agonist is often discontinued on the day of hCG (human chorionic gonadotropin) administration.
Some protocols also might begin GnRH agonist sometime after ovulation in the cycle preceding stimulation in the "mid-luteal" protocol, after the start of menses in the "flare" or "micro-flare" protocol.
GnRH-antagonists (ganirelix acetate or cetrorelix acetate) (Antagon®, Cetrotide®, Orlissa®):
These are other classes of medications used to prevent premature ovulation. They are typically administred several days after stimulation, commonly when the lead follicle if 14 mm or greater, and require fewer injections.
Baseline Pelvic Ultrasound
Around the time of your expected period or shortly thereafter, your medical practitioner will perform an ultrasound scan to examine the ovaries. If your physician detects a cyst, they may withhold further therapy until the cysts resolve . Occasionally, cyst aspiration (drainage) is recommended. This is a procedure in which your doctor inserts a fine needle connected to a syringe, guided by ultrasound, into the cyst. They may also perform a blood test (serum estradiol measurement) to confirm that the ovaries are properly suppressed.
Step 2 - Ovarian Stimulation
In general, ovarian stimulation begins after menstrual bleeding starts. Several similar medications may be used to stimulate follicle development: Lupron®, Gonal-F®, Follistim®, Menopur®, Follistim AQ pen and Gonal-F RFF Pen. GnRH-antagonists (ganirelix acetate or cetrorelix acetate) (Antagon®, Cetrotide®, Orlissa®) are another class of medications used to prevent premature ovulation and, in combination with a GnRH agonist trigger (Lupron®) they may offer protection from severe ovarian hyperstimulation syndrome. GnRh-antagonists are typically used for short periods of time in the late stages of ovarian stimulation. There are several different types of stimulation protocols. Although all protocols more-or-less employ the same types of medications, specific protocols may help certain types of patients to have a better response than other types. It is, however, unrealistic to think that switching from one protocol to another will dramatically change a poorly responding patient to a highly responding patient.
Step 3 - Monitoring of Follicle Development
Follicular development is monitored with a combination of vaginal ultrasound and serum estradiol measurements (blood tests). These tests are performed frequently during the ART cycle, and the dose of medication might be adjusted to improve follicular development. The amount of medication prescribed also depends upon the results of the blood tests and ultrasound exams.
Step 4 - Final Oocyte Maturation with either Leuprolide acetate or hCG Administration
Both leuprolide acetate (Lupron®) and Human chorionic gonadotropin (hCG) (Profasi®, Novarel®, Pregnyl®, Ovidrel®) are hormonal drugs that stimulates the final maturation of the oocytes. Lupron triggers are less likely to cause ovarian stimulation syndrome due to its shorter half-life then hCG. The time of the trigger injection determines when the egg retrieval will be scheduled. Ovulation typically occurs 36-40 hours after the trigger injection of Lupron or hCG.
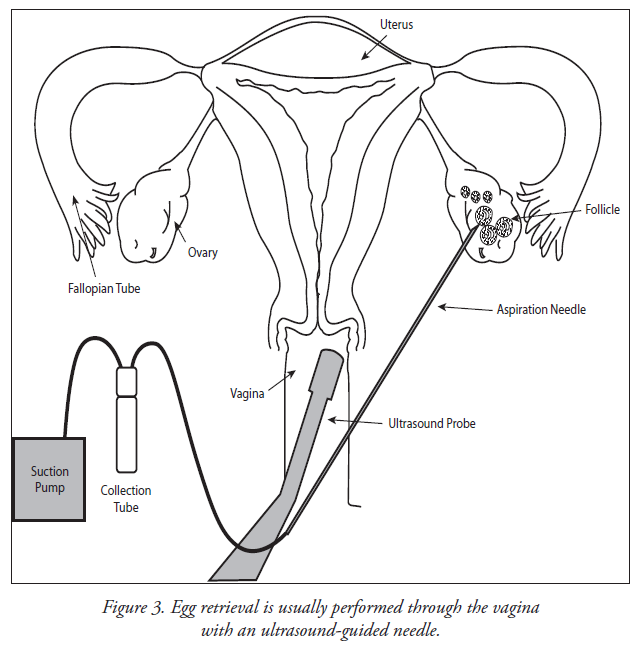
Step 5 - Transvaginal Oocyte Retrieval
- Eggs are removed from the ovary with a needle under ultrasound guidance.
- Anesthesia is provided to make this comfortable.
- Injury and infection are rare.
Oocyte retrieval is performed about 34-36 hours after hCG has been administered. An anesthesiologist usually administers intravenous medications (sedatives and pain relievers) in order to minimize the discomfort that may occur during the procedure. Most patients sleep through the procedure. A transvaginal ultrasound probe is used to visualize the ovaries and the egg-containing follicles within the ovaries. A long needle, which can be seen on ultrasound, can be guided into each follicle and the contents aspirated. The aspirated material includes follicular fluid, oocytes (eggs) and granulosa (egg-supporting) cells. The physician will collect the oocytes and follicular fluid into a test tube and the embryologist will search the follicular fluid and locate the oocytes using a microscope.
After the retrieval, patients recover from anesthesia where they will be observed while the intravenous medications wear off. It is not uncommon to have some vaginal spotting and lower abdominal discomfort for several days following this procedure. Generally, patients feel completely recovered within 1 to 2 days.
The number of oocytes retrieved is related to the number of ovaries, their accessibility, and the number of follicles that develop in response to stimulation. Ultrasound provides only an approximation of the number of oocytes that one can expect to recover. On the average, 8 to 15 oocytes are retrieved per patient.
Step 6 - Semen
In order to obtain sperm of optimal quality, it is important that patients follow clinic instructions for semen collection. (Be aware that clinics may have different protocols for sperm collection for intrauterine insemination and in vitro fertilization. Also, some programs may have men take an antibiotic prior to collection for IVF cases.) Common instructions include:
- Do not ejaculation for at least one to two days but not more than five days before obtaining the semen sample.
- Semen should be collected in a sterile, non-toxic plastic jar provided by the laboratory. Other containers are not acceptable.
- Most clinics prefer that the specimen be collected in the office, if possible. Private collection rooms are usually available. Wash and dry your hands prior to collecting the specimen. Some programs also recommended cleansing the penis followed with rinsing and drying to remove any soap or water.
- Your physician should be notified if there are any problems in collecting. An alternative collection method could be provided, including the use of a special nontoxic condom for collection by sexual intercourse or the use of a vibrator. Commercial condoms cannot be used because they kill the sperm.
- Lubricants should not be used unless directed by a physician.
- Follow the clinic instructions for labeling and transporting the specimen. It is critical to follow these instructions carefully. Keep the jar closed tightly to prevent leakage. Let the lab know if any of the specimen was lost or spilled and whether you have been taking any medications, including herbal remedies.
Masturbation is the most frequent method used to produce a sperm sample. The sample can be produced on the day of oocyte retrieval or prior to the egg retrieval and the sample is frozen. Some physicians recommend freezing a sample as a “back-up” if problems arise in the production or quality of the sperm on the day of egg retrieval.
Occasionally, intercourse using a special condom, electroejaculation, or sperm extraction procedures are required for successful collection.
If a patient is using donor sperm, it will be thawed at the time of the egg retrieval. The donor sperm must be collected and used in accordance with regulatory guidelines.
Step 7- Embryology Lab Procedures
After eggs are retrieved, they are transferred to the embryology laboratory where they are kept in conditions that support their needs and growth. The embryos are placed in small dishes or tubes containing "culture medium," which is special fluid developed to support development of the embryos made to resemble that found in the fallopian tube or uterus. The dishes containing the embryos are then placed into incubators, which control the temperature and atmospheric gasses the embryos experience.
A few hours after eggs are retrieved, sperm are placed in the culture medium with the eggs, or individual sperm are injected into each mature egg in a technique called intracytoplasmic sperm injection (ICSI).
The ICSI technique has been developed to treat cases of severe male factor infertility. The ICSI technique attempts to achieve fertilization by the injection of a single sperm into the cytoplasm (interior) of the egg. Mature eggs are freed of surrounding cells by a combination of enzyme treatment and microdissection. Using special micromanipulation equipment (joystick-controlled robotics), the eggs are individually injected with a single sperm. Injected eggs are returned to the laboratory incubator and are treated as in conventional IVF-ET.
The eggs are then returned to the incubator, where they remain to develop. Periodically, over the next few days, the dishes are inspected so the development of the embryos can be assessed.
The following day after eggs have been inseminated or injected with a single sperm (ICSI), they are examined for signs that the process of fertilization is underway. At this stage, normal development is evident by the still single cell having two nuclei; this stage is called a zygote. Two days after insemination or ICSI, normal embryos have divided into about four cells. Three days after insemination or ICSI, normally developing embryos contain about eight cells. Five to seven days after insemination or ICSI, normally developing embryos have developed to the blastocyst stage, which is typified by an embryo that now has 80 or more cells, an inner fluid-filled cavity, and a small cluster of cells called the inner cell mass.
It is important to note that since many eggs and embryos are abnormal, it is expected that not all eggs will fertilize and not all embryos will divide at a normal rate. The chance that a developing embryo will produce a pregnancy is related to whether its development in the lab is normal and whether it is genetically competent. Embryo morphology is assessed and graded by embryologists.
Despite reasonable precautions, any of the following may occur in the lab that would prevent the establishment of a pregnancy:
- Fertilization of the egg(s) may fail to occur.
- One or more eggs may be fertilized abnormally, resulting in an abnormal number of chromosomes in the embryo; these abnormal embryos will not be transferred.
- The fertilized eggs may degenerate before dividing into embryos, or adequate embryonic development may fail to occur.
- Bacterial contamination or a laboratory accident may result in loss or damage to some or all of the eggs or embryos.
- Laboratory equipment may fail, and/or extended power losses can occur which could lead to the destruction of eggs, sperm and embryos.
- Other unforeseen circumstances may prevent any step of the procedure to be performed or prevent the establishment of a pregnancy.
Embryos are often transferred to the uterus most commonly 5 days after the egg retrieval but can be from three to six days after retrieval.
Preimplantation Genetic Diagnosis (PGD) and Preimplantation Genetic Testing (PGT) are two techniques that can be used during in vitro fertilization (IVF) procedures to test embryos for genetic disorders prior to their transfer to the uterus. PGD makes it possible for couples or individuals with serious inherited disorders to decrease the risk of having a child who is affected by the same problem. Both PGD and PGT involve the use of the micromanipulator to remove a cell from an embryo. This cell is then sent to a diagnostic lab to determine the embryo’s genetic competency. Acceptable embryos can then be transferred into the patient, with the goal of increasing the probability of having a successful pregnancy and decreasing her odds of having a child affected with a genetic condition.
Step 8 - Embryo Transfer
- After 2-6 days of development, the best appearing embryo(s) is selected for transfer.
- The number chosen influences the pregnancy rate and the multiple pregnancy rate.
- A woman’s age, the genetic competency, and the appearance of the developing embryo have influences on pregnancy outcome.
- Embryos are placed in the uterine cavity with a thin tube.
- Excess embryos of sufficient quality that are not transferred can be frozen.
The embryo transfer procedure is usually performed three to five days after oocyte retrieval is a fresh embryo transfer. It is more common to cryopreserve embryos during a fresh embryo transfer and the transfer embryos into the uterus during a separate month during a frozen embryo transfer.
The physician will pass a catheter gently through the cervix into the uterus and deposit the embryo(s) into the uterine cavity along with an extremely small amount of fluid. This procedure usually does not require anesthesia, and the patient usually leaves the office after a brief recovery period. SART has specific guidelines on the number of embryos to transfer. Ideally, single embryo transfer occurs.
Step 9 - Cryopreservation of Embryos
Embryo freezing is an important part of the IVF process. Patients who have additional good quality embryos can freeze them for future use. These embryos provide a second or even third opportunity for pregnancy without undergoing another ovarian stimulation and retrieval.
Embryos that meet developmental criteria for appearance and rate of growth can be frozen at any of several stages of development. There are 2 ways to freeze embryos. One is called slow cooling. With this method embryos are placed into special freezing solutions, and using a computer, the temperature of the embryos is slowly decreased. Frozen embryos are then stored in liquid nitrogen (at -196°C or approximately -400°F), or sometimes, in liquid nitrogen vapor.
A more recent and more common technique for freezing embryos is called vitrification. In this ultra-rapid freezing method, embryos are placed into special solutions and then placed immediately into liquid nitrogen. The method used to freeze embryos dictates how the embryos must be warmed or thawed. Not all embryos survive the freezing/thawing procedure and sometimes an embryo cannot be found after freezing.
Embryos can be transferred into patients whose cycle has been synchronized with that of the stage of the frozen embryo. Alternatively, embryos can be transferred during a “natural” cycle. Embryos can be stored indefinitely without a compromise in their quality.
Step 10 – Hormonal Support of the Uterine Lining (Progesterone Supplements)
- Successful attachment of embryo(s) to the uterine lining depends on adequate hormonal support. In a fresh embryo transfer, progesterone is routinely given.
- In a medicated frozen embryo transfer, estradiol and progesterone supplementation are given.
- Hormone support is given until the pregnancy test. If the pregnancy test is positive, the hormone support is typically continued until 9-12 weeks of gestational age if the pregnancy test is positive.
Step 11 - Pregnancy Test
A pregnancy test is necessary regardless of vaginal spotting or bleeding. It determines if pregnancy has occurred and is typically done 8-14 days after the embryo transfer. If the pregnancy test is negative, hormone supplementation is stopped. If the pregnancy test is positive, hormone supplementation is continued.
Step 12 - Early Pregnancy Follow-up
Close scrutiny of a pregnancy is necessary to try to identify miscarriages or ectopic pregnancies and to help counsel regarding the status and treatment of multiple gestations. Patients are generally released to their obstetrician at 8-10 weeks gestation.